


TEF-Guard Buy 5 Get 1 Free!
Only for a limited time:
- Cytoflex Textured Tef-Guard
- Cytoflex Ti-Enforced Tef Guard
Order Now

When using it, peel off blister Tyvek film. Holding S1 vial firmly, remove the cap. Dispense the granules of S1 into a sterile container.
After exposure the bony defect with mucoperiosteal flap, completely remove the granulation tissue and inflammatory tissue.
When opening the sterile package, never store remained product.
Put saline solution in the bone graft material that has been placed in the sterilized container.
Use the recommended weight and amount of saline for successful use:
1) Please keep the following points in mind!
· Please comply with the recommended allowance
· Do not divide the product for multiple uses
· Mix with saline solutions well enough
2) The amount of saline is most important!
· Place S1 in the tray and hydrate the materials with saline
· Please use the recommended amount of saline only.
· Do not soak in saline after shaping for surgery.
3) It is important to knead S1 evenly!
· Knead the dough enough for at least 30 seconds by using hands or tools to form a lumpy shape before using S1.
After making the product in a paste form suitable for the defect area, apply it to the surgical site using a dental instrument and press it down.
After filling the graft, cover the surgical site with mucoperiosteal flap and should be fixed by sutures. Be sure to completely seal the implantation site to prevent exposure.
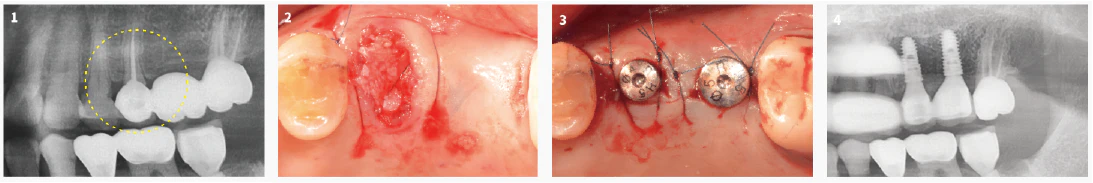
Alveolar Ridge Preservation without Membrane
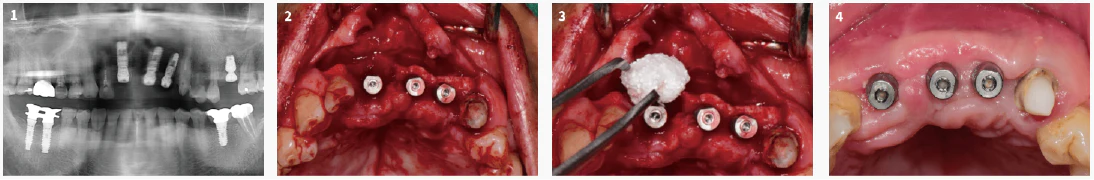
Moldable Augmentaion in Anterior Area
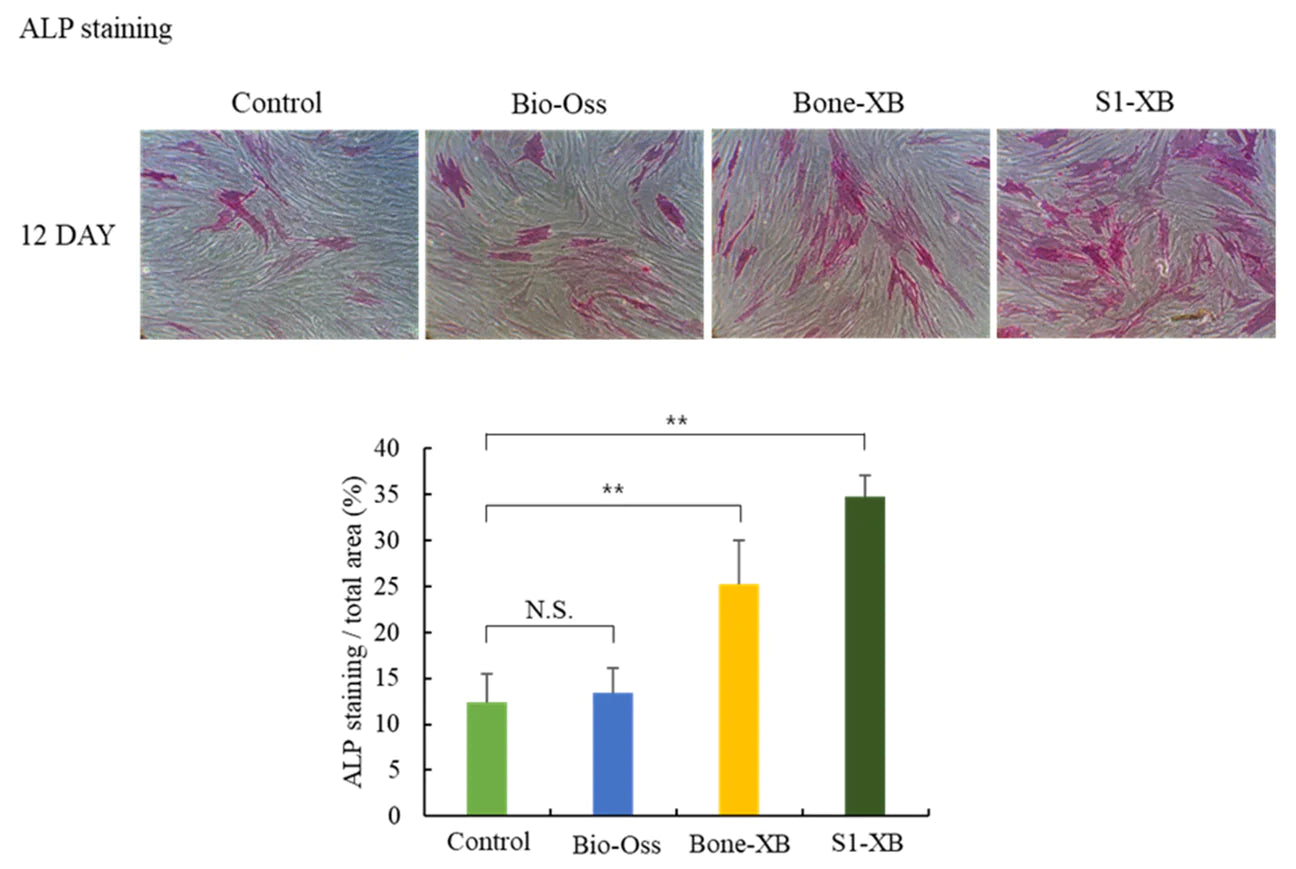
Independent research shows S1-XB extract significantly promoted osteoblast differentiation.
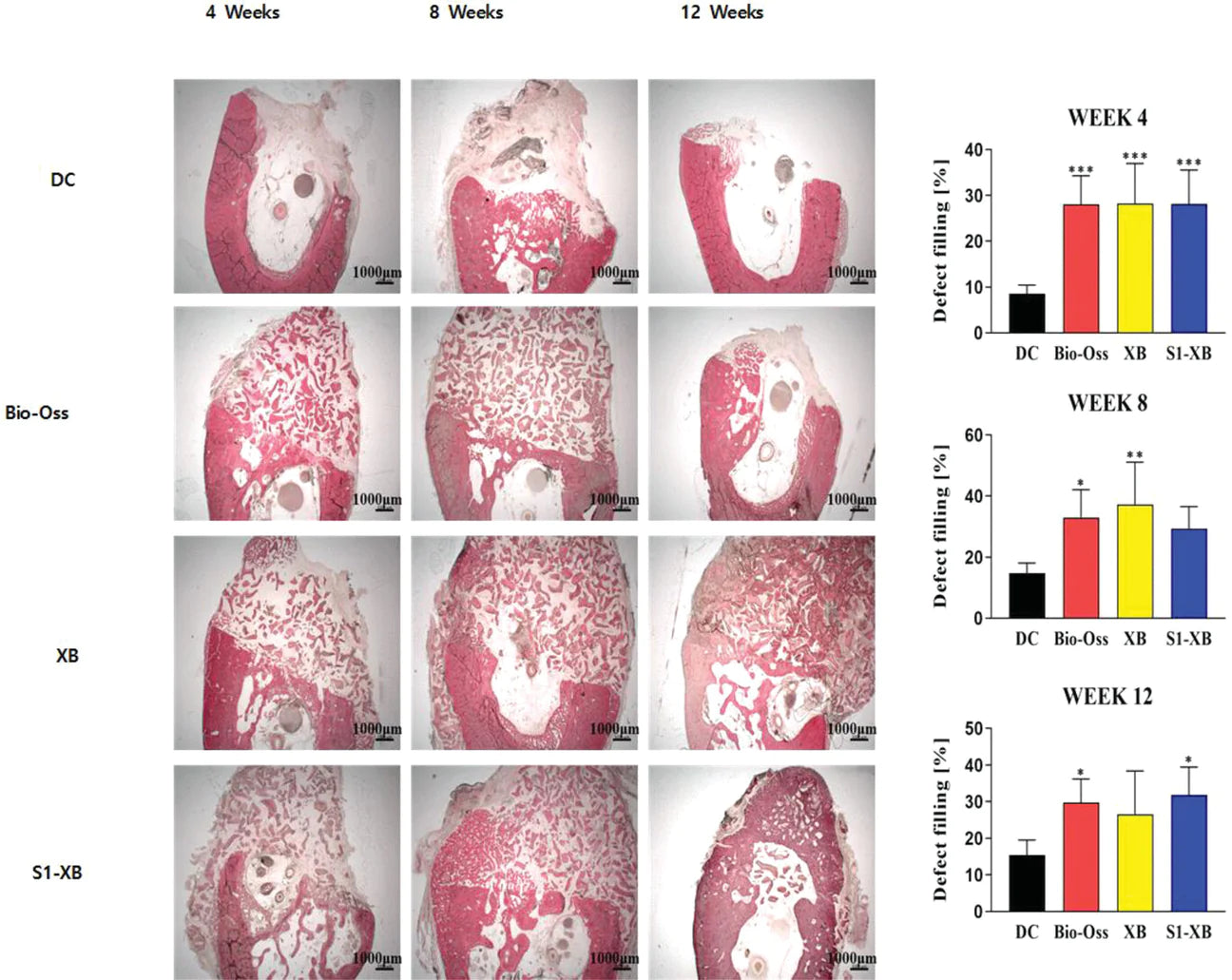
The results of radiography, micro-CT, and histological analysis showed S1-XB effectively regenerated bone, indicated by the increase in newly formed bone and bone microstructure thereby improving bone defect filling in the critical defect model and equivalent to the Bio-Oss.
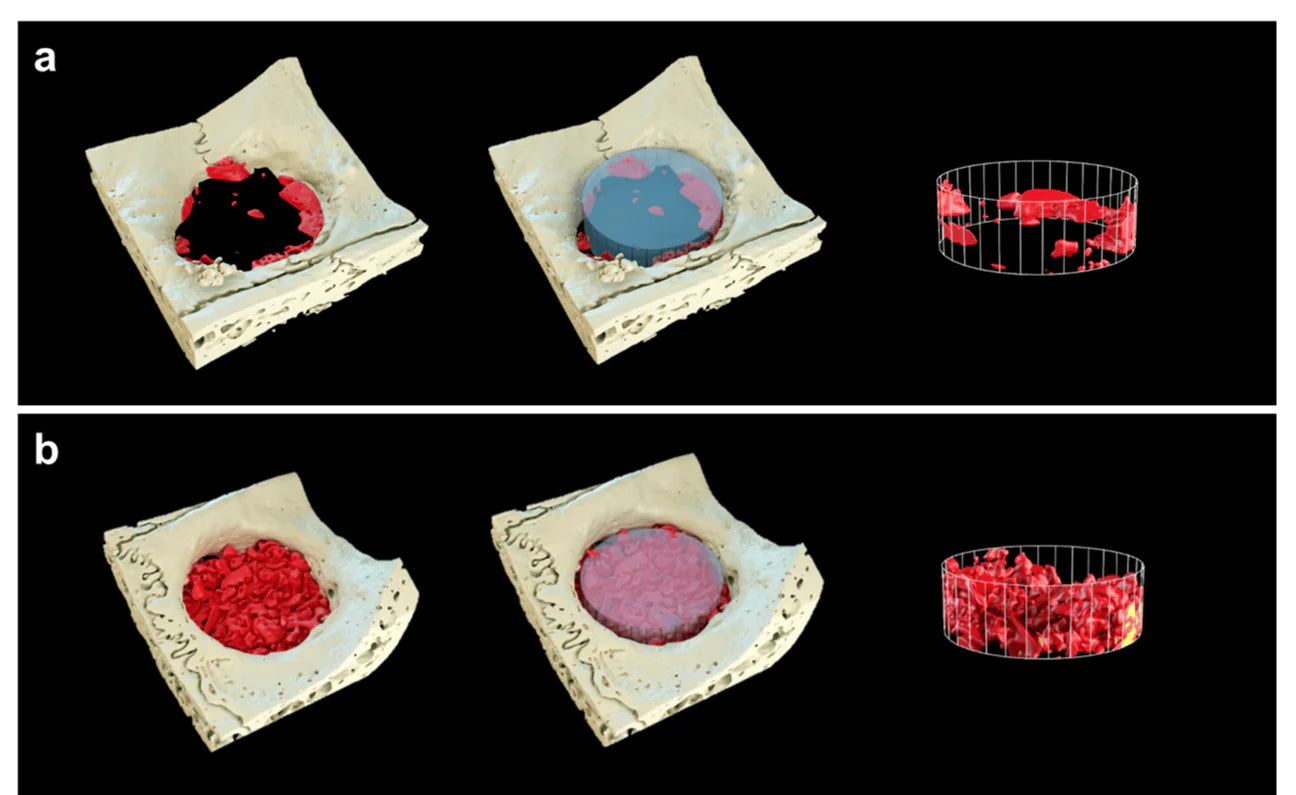
Independent research shows higher bone regeneration in the defects treated with the S1 compare to the control group and bone graft material was easily moldable with the desired shape during surgery.
Reconstructed three-dimensional (3D) images within the 6 mm region of interest using micro-CT analysis after 8 weeks of healing. (a) Control, (b) S1 (yellow: xenograft materials, red: newly formed bone, scale bars = 6 mm).
A.) Do not use if package is opened or damaged or if expiration date has been exceeded.
B.) This product should only be used by trained dentists or oral surgeons.
A.) Patients with osteomyelitis
B.) Patients with severe liver dysfunction
C.) Patients with severe cardiac dysfunction
A. In general, the conditions considered as standard contraindications for bone graft are metabolic diseases, osteoporosis, steroid therapy, autoimmune diseases, nicotinism.
B. As S1 derived from bovine cancellous bone, S1 must not be given to patients allergic to bovine bone. (Although S1 meets “ISO 10993-10 Test for irritation and skin sensitization”, allergic reaction may occur in someone with S1 sensitivity.)
C. For bone regeneration, the product is only implantable to bone tissue that is directly connected to living bone tissue and host bone. Experience has shown that movement due to increased physical loads (compression loads) or implantation of implants (2-step procedures) should be avoided until several weeks after insertion of the product. Experiments have shown that the physical loading (compression load) of this product augmented areas is possible after 6 months at the earliest. Implant placement time is determined by the amount of remaining local bone.